Gibbs Free Energy. 2 free energy and chemical change. The gibbs free energy of a system at any moment in time is defined as the enthalpy of the system minus the product of the temperature times the entropy of the system.
Gibbs energy or gibbs function; Gibbs free energy is a concept invented to create a thermodynamic relationship between enthalpy and entropy. Gibbs free energy is also known as (g), gibbs' free energy, gibbs energy, or gibbs function. He begins by using three spontaneous reactions to explain how a change in enthalpy. Gibbs free energy and chemical reactions:
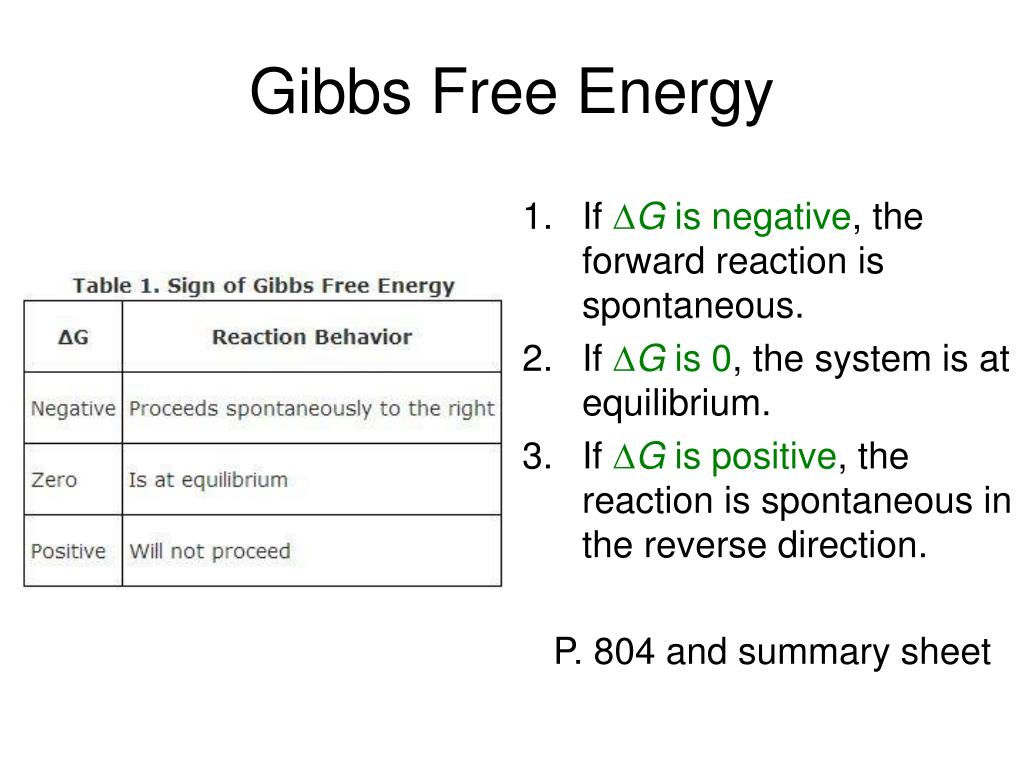
Intuition behind why spontaneity is driven by enthalpy, entropy and temperature.
Thus, gibbs free energy is most useful for thermochemical processes at constant temperature and pressure: Paul andersen attempts to explain gibbs free energy. Dexter perkins (university of north dakota), andrea koziol (university of dayton), john brady (smith college). The free energy signifies the maximum energy available, or free, to do useful work. Gibbs free energy and other thermodynamic functions. Gibbs free energy is a concept invented to create a thermodynamic relationship between enthalpy and entropy. Josiah willard gibbs, an american mathematician, first described gibbs free energy in the 1870s. Other articles where gibbs free energy is discussed: The standard gibbs free energy is calculated using the free energy of formation of each component of a reaction at standard pressure. In thermodynamics, the gibbs free energy (or gibbs energy) is a thermodynamic potential that can be used to calculate the maximum reversible. That means energy that isn't dissipated through heat or expansion of the system. If you have already read the page about how to do this with total. The gibbs free energy g is defined by.
That means energy that isn't dissipated through heat or expansion of the system. In thermodynamics, the gibbs free energy (or gibbs energy) is a thermodynamic potential that can be used to calculate the maximum reversible. Intuition behind why spontaneity is driven by enthalpy, entropy and temperature. Gibbs free energy is also known as (g), gibbs' free energy, gibbs energy, or gibbs function. Gibbs free energy thermodynamic potentials internal energy u(s,v) helmholtz free energy a(t,v) gibbs energy is also the chemical potential that is minimized when a system reaches equilibrium at.

Entropy, temperature, volume, and pressure.
Paul andersen attempts to explain gibbs free energy. Gibbs free energy is the energy available in a system to do work (reversibly, if you want to be nitpicky). Gibbs free energy g provides a means of defining equilibrium or of the tendency of a reaction to proceed in a given direction. The american physicist josiah gibbs introduced (ca. The free energy signifies the maximum energy available, or free, to do useful work. In thermodynamics, the gibbs free energy (or gibbs energy) is a thermodynamic potential that can be used to calculate the maximum reversible. The gibbs free energy g is defined by. If you have already read the page about how to do this with total. Entropy, temperature, volume, and pressure. Gibbs free energy and other thermodynamic functions. That means energy that isn't dissipated through heat or expansion of the system. Dexter perkins (university of north dakota), andrea koziol (university of dayton), john brady (smith college). The standard gibbs free energy is calculated using the free energy of formation of each component of a reaction at standard pressure.
Dexter perkins (university of north dakota), andrea koziol (university of dayton), john brady (smith college). The classical carnot heat engine. It is given by the equation: I.e., does the reaction almost always go in one direction. Other articles where gibbs free energy is discussed:

The free energy signifies the maximum energy available, or free, to do useful work.
Standard free energy change, dgo —the standard free energy change, dgo can be calculated (1) by substituting standard enthalpies and entropies of reaction and a kelvin temperature into the gibbs. Gibbs energy is the maximum (or reversible) work that a thermodynamic system can perform at a constant temperature and we can mathematically define the gibbs free energy by the equation 1875) a thermo‐dynamic quantity combining enthalpy and entropy into a single value called free energy (or gibbs free energy). Gibbs free energy and chemical reactions: Gibbs free energy is that thermodynamic quantity of a system the decrease in whose value during a process is equal to the maximum possible useful work that can be obtained from the system. Dexter perkins (university of north dakota), andrea koziol (university of dayton), john brady (smith college). The gibbs free energy of a system at any moment in time is defined as the enthalpy of the system minus the product of the temperature times the entropy of the system. The free energy signifies the maximum energy available, or free, to do useful work. Gibbs free energy g provides a means of defining equilibrium or of the tendency of a reaction to proceed in a given direction. It is given by the equation: Gibbs energy or gibbs function; Ascertaining the gibbs free energy of a system offers a way to determine whether one arrow is much larger than the other; The important role of temperature.
0 komentar:
Posting Komentar